Notes to Rock Magnetism Basics: Magnetic Minerals
Titanomagnetite as a recorder of the paleomagnetic field
Magnetism of rocks is very weak and yet it retains the information of the past geomagnetic field millions of years ago. This memory effect is similar to that of the magnetic stripe on plastic cards.
Main magnetic mineral contained in volcanic rocks is titanomagnetite which is a solid solution of magnetite (Fe3O4) and ulvospinel (Fe2TiO4). Therefore, titanomagnetite is Fe3-xTixO4 where x is a content of ulvospinel. The left figure below shows a titanomagnetite grain with x = 0.47 contained in andesite lava flow in the Ontake Volcano, Japan. The image was taken by a scanning electron microscope (SEM). Such a large homogeneous grain, though conspicuous in the SEM image, is magnetically unstable and has no contribution to the stable remanence because only a small grain of ~1μm or less can hold magnetic memory. In the figure, some fine titanomagnetite grains are also recognized, which must be the main carrier of the stable remanence.
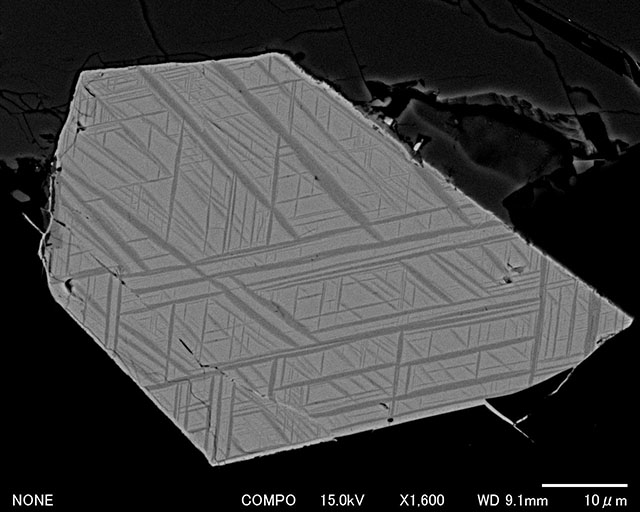
Fine titanomagnetite grains with skeletal form are often crystallized in rapid cooled magma as shown in the right figure above, which were found in basalt lava flow in Iceland. Owing to its small size of each grain, skeletal titanomagnetite can carry a stable remanence. Furthermore, in the case of this lava flow, each grain is divided into even smaller regions by trellis-type structure called lamellae.
Titanomagnetite with trellis-type lamellae is formed as a result of high-temperature (deuteric) oxidation during the initial cooling of the solidified magma from ~1000°C to ~500°C. High-temperature oxidation is the process of exsolution in which one phase titanomagnetite (β-phase) turns into two phases with newly created titanium-rich α-phase. The α-phase is a solid solution of ilmenite (FeTiO3) and hematite (Fe2O3) (usually close to ilmenite) and the host β-phase is now depleted in titanium (close to magnetite). As shown in right figure (SEM image of Icelandic basalt lava), high-temperature oxidation produces small regions of titanium-poor β-phase surrounded by exsolved titanium-rich α-phase. Hence, volcanic rocks lacking in fine magnetic grains are still able to keep a stable remanence once they underwent high-temperature oxidation. High-temperature oxidation depends on temperature and partial pressure of oxygen within the magma, but the simplest explanation of the process is as the following for the case of x = 0.5:
Fe2.5Ti0.5O4 + \(\frac{1}{12}\)O2 \(\longrightarrow\) \(\frac{2}{3}\)Fe3O4 + \(\frac{1}{2}\)FeTiO3 \(\quad\) (1)
There is another type of oxidation called low-temperature oxidation which proceeds under 200~300°C. This process turns titanomagnetite (β-phase) into titanomaghemite which is a cation-deficient phase (\(\gamma\)-phase). In the low-temperature oxidation, some of Fe2+ ions are oxidized into Fe3+. One third of those turned into Fe3+ are removed by diffusion, producing vacancies while the cubic crystal structure is retained. The following is a case for x = 0.5 when oxidation level is 0.6 (i.e., 60\(\%\) of Fe2+ is turned into Fe3+),
Fe\(^{3+}\)Fe\(^{2+}_{1.5}\)Ti\(^{4+}_{0.5}\)O\(^{2-}_4\) \(\longrightarrow\) Fe\(^{3+}_{1.6}\)Fe\(^{2+}_{0.6}\)Ti\(^{4+}_{0.5}\)\(\Box_{0.3}\)O\(^{2-}_4\) + 0.3Fe\(^{3+}\) \(\quad\) (2)
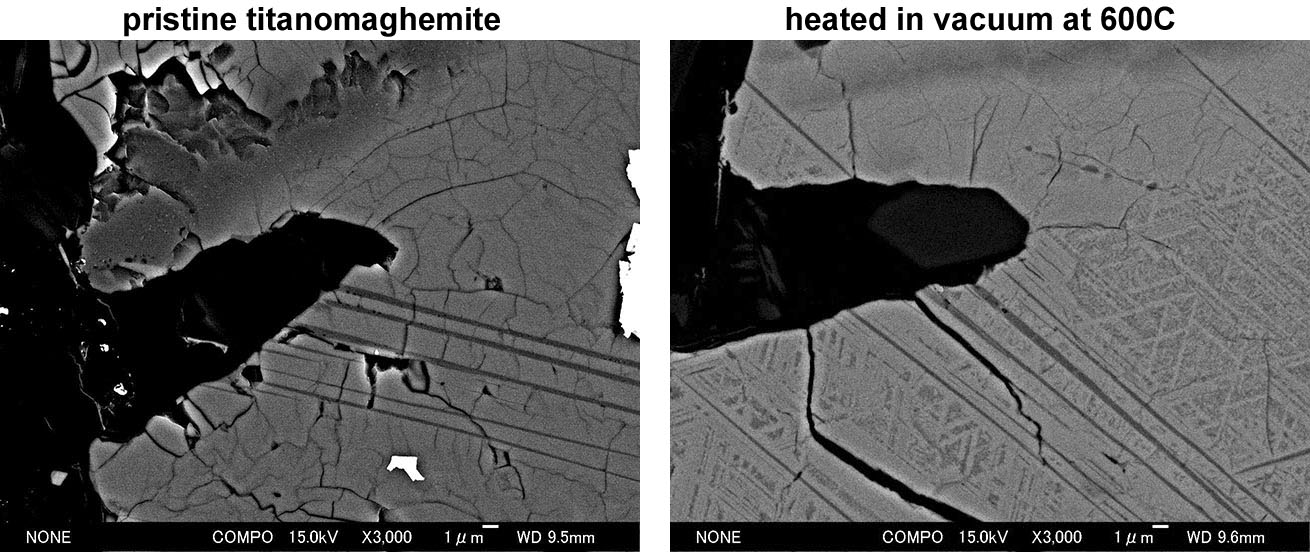
Another problem of titanomaghemite is that it easily breaks down during laboratory heat treatment at low temperatures around 300°C. As the inversion products include magnetite, strong stray remanence usually emerges. When the above mentioned andesite lava (left figure above) was heated to 600°C in vacuum, the cracked surface of titanomaghemite turned into trellis-type structure (right figure above). Although the grain surface is similar to that of high-temperature oxidized titanomagnetite, the process involved is completely different. The inversion process for the titanomaghemite shown in (2) (x = 0.5 with oxidation level of 0.6) is given by the following formula.
Fe2.2Ti0.5\(\Box\)0.3O4 \(\longrightarrow\) \(\frac{3}{5}\)Fe3O4 + \(\frac{1}{5}\)Fe2O3 + \(\frac{1}{2}\)TiO2 \(\quad\) (3)
Reference:
- Tanaka, H., and Y. Yamamoto, Microscopic observation of titanomagnetite grains during palaeointensity experiments of volcanic rocks, Geophys. J. Int., 196, 145-159, 2014. (→ doi:10.1093/gji/ggt387)